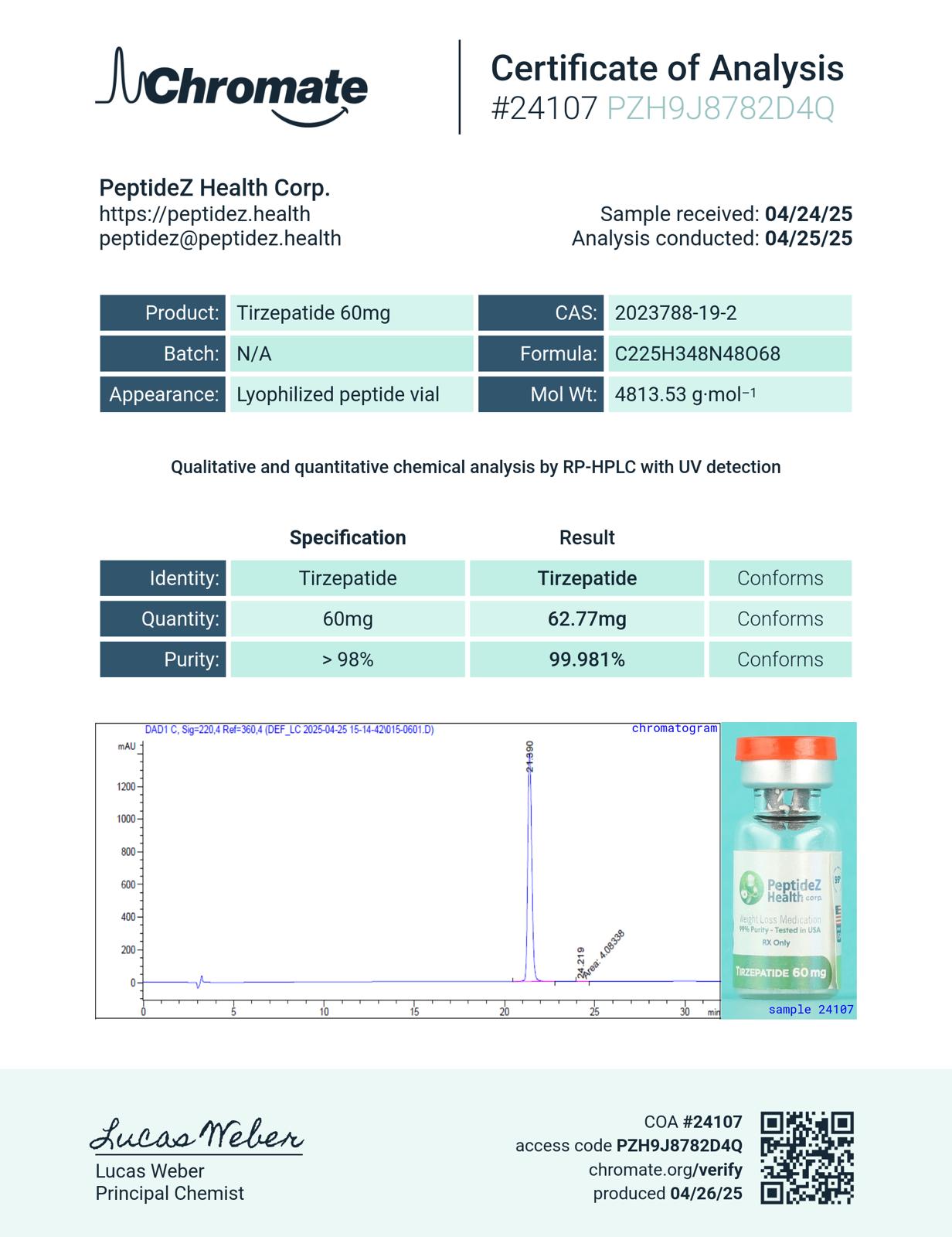
Tirzepatide 60mg
Tirzepatide 60mg is a dual GIP/GLP-1 receptor agonist designed to support effective weight management and metabolic health. It works by regulating appetite, improving insulin sensitivity, and promoting fat oxidation, making it ideal for patients seeking gradual yet sustainable body transformation.
Tirzepatide: A Dual GIP/GLP-1 Receptor Agonist for Weight Management and Glycemic Control

Tirzepatide is an innovative prescription medication developed by Eli Lilly and Company.
It functions as a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist, offering a unique mechanism of action that addresses both metabolic health and weight management.
Approved by the U.S. Food and Drug Administration (FDA) for the treatment of adults with type 2 diabetes, tirzepatide has also demonstrated significant efficacy in promoting weight loss, making it one of the most promising therapies in modern endocrinology and obesity medicine.
How Does Tirzepatide Work?
Tirzepatide mimics the natural actions of two key gut hormones involved in regulating metabolism and appetite: GIP and GLP-1 . These hormones are released after eating and work together to:
- Increase insulin secretion when blood sugar levels are high.
- Reduce the amount of glucose produced by the liver.
- Slow gastric emptying, which prolongs digestion and enhances satiety.
- Suppress appetite through central nervous system effects.
By activating both GIP and GLP-1 receptors simultaneously, tirzepatide provides a more comprehensive metabolic response than medications that target only one of these pathways. This dual action not only improves glycemic control but also contributes significantly to weight reduction.
Administration and Dosage
Tirzepatide is administered once weekly via subcutaneous injection using a pre-filled pen (autoinjector). The available dosage strengths include 5 mg, 10 mg, 15 mg, and 20 mg per 0.5 mL , allowing for flexible titration based on individual patient needs and tolerability. The gradual increase in dose helps minimize gastrointestinal side effects commonly associated with incretin-based therapies.
This convenient dosing schedule improves adherence and makes tirzepatide an attractive option for long-term use in chronic conditions such as type 2 diabetes and obesity.
Clinical Efficacy: Glycemic Control and Weight Loss
Clinical trials have demonstrated that tirzepatide is highly effective in improving blood sugar levels among patients with type 2 diabetes. When used alongside diet and exercise, it helps regulate glycated hemoglobin (HbA1c), contributing to better overall disease management.
In addition to its antidiabetic benefits, tirzepatide has shown remarkable results in clinical studies focused on weight loss. In the pivotal SURMOUNT-1 trial (NCT04184622) , participants receiving tirzepatide achieved substantial reductions in body weight over a 72-week period:
- 5 mg/week: Average weight loss of 16.1 kg (35.5 lbs)
- 10 mg/week: Average weight loss of 22.2 kg (48.9 lbs)
- 15 mg/week: Average weight loss of 23.6 kg (52.0 lbs)
- Placebo group: Average weight loss of 2.4 kg (5.3 lbs)
These findings highlight tirzepatide’s potential as a transformative therapy for individuals seeking meaningful and sustained weight reduction.
Regulatory Approval and Availability
Tirzepatide has been approved by major regulatory agencies worldwide, including the U.S. FDA and ANVISA (Brazil’s National Health Surveillance Agency) . In Brazil, it was granted registration under MS No. 1.1260.0202 on September 25, 2023, following Resolution RE No. 3,591 of September 21, 2023. It is currently available as single-dose pens in various strengths, ensuring ease of administration and accurate dosing.
The drug must be prescribed and monitored by a licensed healthcare provider and should always be used as part of a comprehensive treatment plan that includes dietary changes, physical activity, and regular medical follow-up.
Important Safety Information
As with any medication, tirzepatide carries some risks and contraindications. It is not recommended for individuals with a history of pancreatitis or those diagnosed with type 1 diabetes . Patients should be closely monitored for adverse effects, particularly during the initial stages of treatment.
Common side effects may include nausea, vomiting, diarrhea, and decreased appetite—most of which tend to diminish over time. It is essential to follow the instructions provided by your physician or pharmacist carefully and report any unusual symptoms immediately.
Compliance with International Standards
All documentation related to tirzepatide complies with current Brazilian legislation, including Law No. 6,360/1976 , Decree No. 8,077/2013 , Law No. 9,787/1999 , and RDC Resolution No. 200/2017 , among other relevant regulations. While technical opinions remain unchanged after approval, critical information regarding formulation, storage, shelf life, and usage guidelines can be accessed via official sources.
Patients are encouraged to review the full prescribing information and consult their healthcare provider before initiating treatment.
Final Considerations
Tirzepatide represents a major advancement in the treatment of type 2 diabetes and obesity. Its dual-action mechanism, combined with a once-weekly dosing schedule and proven efficacy in clinical trials, makes it a powerful tool for improving metabolic health and achieving sustainable weight loss.
However, it is crucial to emphasize that tirzepatide is not a standalone solution. For optimal outcomes, it should be integrated into a broader lifestyle strategy involving healthy eating, regular physical activity, and continuous medical supervision.
If you believe tirzepatide could be right for you, speak with your doctor to determine whether this medication aligns with your health goals and medical history.
Scientific Backing & Quality Assurance
- Formulated with 99% purity peptides and bacteriostatic solution
- Complies with international standards and clinical protocols
- Manufactured under strict quality control for safe and effective use
Fast Delivery
- Standard delivery within 3 business days in the United States
- International shipping available – import times may vary by country
- Secure packaging and real-time tracking included
Treatment Support – Risk & Side Effect Management
- Personalized guidance during your treatment cycle
- Medical support to manage potential adverse effects
- Continuous monitoring advice for a safer experience